Activities & Operations
DCT approaches

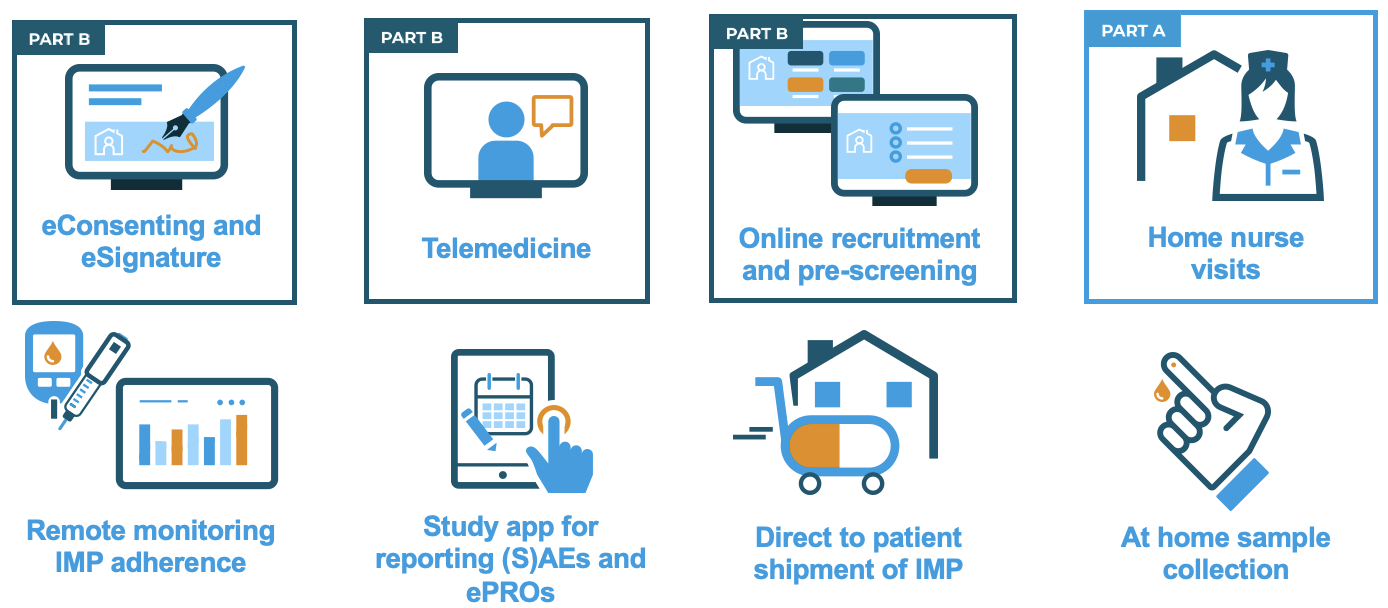
Several approaches have been used in DCTs to move trial activities towards participants’ homes. A selection of these, which were used in RADIAL, are shown in the figure below. Others include postal or telephone contact, shipment of study materials to participants, use of local healthcare services and routine care, use of centralised healthcare databases to identify trial outcomes and wearable devices. Different approaches will be appropriate for different trials and different participant populations and it is important to select the best approach carefully.
Recommendations
How Trials@Home reached these recommendations
We conducted a systematic review to identify best practices within DCTs, in addition to interviews with stakeholders involved in DCT case studies. RADIAL also brought in-depth experience with multiple DCT approaches. This work identified some of the challenges and solutions regarding methods used in DCTs.
Further reading
Publications
Learning from Remote Decentralised Clinical Trial (RDCT) experiences: a qualitative analysis of interviews with trial personnel, patient representatives and other stakeholders.
Coyle, et al |
British Journal of Clinical Pharmacology |
2021 |
|---|
A Systematic Review of Methods used to Conduct Decentralised Clinical Trials.
Rogers, et al |
British Journal of Clinical Pharmacology |
2021 |
|---|
A secondary qualitative analysis of stakeholder views about participant recruitment, retention, and adherence in decentralised clinical trials (DCTs).
Coyle, et al |
Trials |
2022 |
|---|
Bringing trial activities to participants – The Trials@Home RADIAL proof-of-concept trial on decentralisation.
Zuidgeest, et al |
Clinical Pharmacology & Therapeutics |
2025 |
|---|
Regulatory Interactions and Learnings – RADIAL the Trials@Home Proof-of-Concept Trial on Decentralization.
Gardarsdottir, et al |
Clinical Pharmacology & Therapeutics |
2025 |
|---|
Selecting and Preparing Clinical Sites for the Successful Conduct of Decentralized Clinical Trial Activities – Findings From the Trials@Home RADIAL Proof-of-Concept Trial.
Lipinska, et al |
Clinical Pharmacology & Therapeutics |
2025 |
|---|
Recruiting and Consenting Decentralized Clinical Trial Participants – Learnings from the Trials@Home RADIAL Proof-of-Concept Trial.
Lagerwaard, et al |
Clinical Pharmacology & Therapeutics |
2025 |
|---|
The Supply of Investigational Medicinal Product and Management of Study Materials for Decentralized Participants – Insights from the Trials@Home RADIAL Proof-of-Concept Trial.
Heath, et al |
Clinical Pharmacology & Therapeutics |
2025 |
|---|
Operationalising Decentralised Clinical Trials: Technology Insights from the Trials@Home RADIAL Proof-of-Concept Trial.
Hanke, et al |
Clinical Pharmacology & Therapeutics |
2025 |
|---|
A cross-sectional survey on the early impact of COVID-19 on the uptake of decentralised trial methods in the conduct of clinical trials.
Suman, et al |
Trials |
2022 |
|---|
