Objectives and key deliverables

The six work packages (WPs) of Trials@Home and their interdependencies.
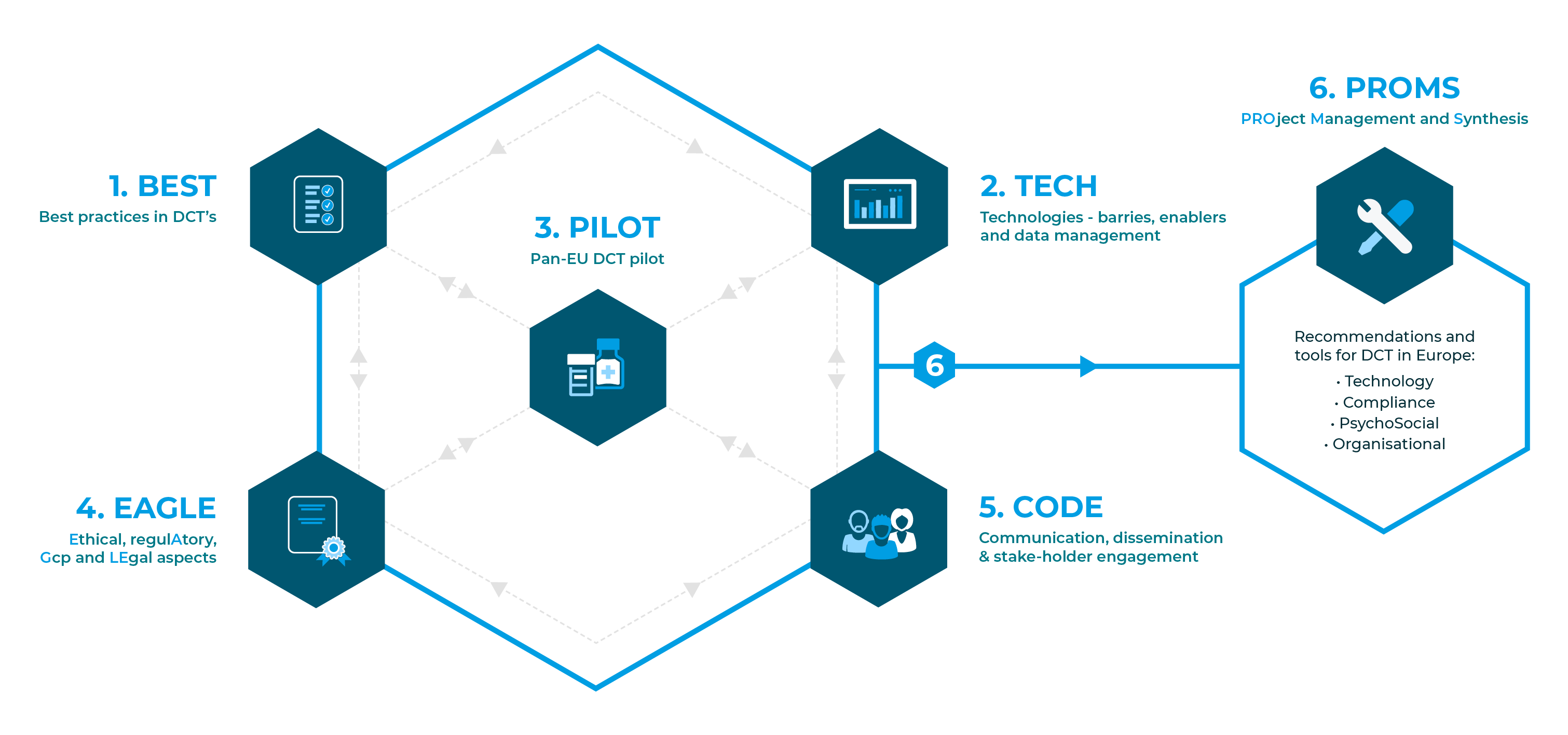
1. Best practices in DCTs
WP1 BEST had three objectives:
- To collect and analyse prior and ongoing experiences with DCTs;
- To provide best practices, selection criteria and recommendations for implementation into the WP3 PILOT.
- To provide updates to final recommendations based on ongoing case studies conducted in parallel to the pilot.
Deliverables |
Timeline |
Status |
|---|---|---|
| D1.1 First set of recommendations for DCTs (to be implemented in the pan-EU pilot DCT) |
M12 | Completed |
| D1.2 Criteria for selection of appropriate trials |
M12 | Completed |
| D1.3 Updated of recommendations based on ongoing case studies running in parallel to the WP3 pilot |
M66 | Completed |
2. Technologies – barries, enablers and data management
WP2 TECH had the following objectives:
- To scan for and identify a wide range of potential technologies and technological solutions (i.e., software packages that implement certain technologies) that support DCTs, either available as marketed products or as a validated proof-of-concepts.
- To perform a quality assessment and prioritisation of the identified technological solutions, including evaluation of technologies available as proof-of-concept not validated specifically for use in clinical trials.
- To develop recommendations for evaluated technological solutions.
- To refine these recommendations, and select and tailor the appropriate technology package for the WP3 pan-EU pilot DCT, based on chosen therapeutic areas and study protocol.
- To design, assemble and test the infrastructure for the selected technology package for the WP3 pan-EU pilot DCT.
- To provide technical support for the training in, and use of the resulting composition platform to those parties involved in the clinical trial.
- To define data quality, management, communication, and activity flows.
Deliverables |
Timeline |
Status |
|---|---|---|
| D2.1 Glossary of terms and definitions used within WP2 (task 2.1.1) |
M6 | Completed |
| D2.2 Detailed list of quality assessment criteria and assessment procedures (task 2.1.2) |
M12 | Completed |
| D2.3 Technology scan: list of technologies suitable for DCTs for each of the building blocks of an DCT to be used in the quality assessment (task 2.1.1) |
M12 | Completed (confidential) |
| D2.4 Technology package for the pan-EU pilot study including data flows and connections with external systems where necessary (task 2.3) |
M17 | Completed (confidential) |
| D2.5 Pilot data management plan and operational database connected with the central platform and other systems tailored to the needs of the pan-EU pilot and with data export suitable for statistical analysis (task 2.4) |
M42 | Completed (confidential) |
| D2.6 An interconnected, fully tested technology platform that connects the technological solutions chosen for the technology package and supports the WP3 pan-EU Pilot (task 2.5) |
M47 | Completed |
| D2.7 Technology support system (task 2.6) |
M47 | Completed |
| D2.8 Updated summary and detailed report with recommendations for integrated technologies to be used in DCT and hybrid approaches, based on pilot findings (task 2.7) |
M66 | Completed |
3. Pan-EU DCT pilot
WP3 PILOT had the following objectives:
- To design, set-up and run a pan-EU pilot, comparing traditional clinical trial approaches to fully and hybrid DCT approaches.
- To comparatively analyse the components (traditional clinical trial and hybrid and/or fully DCT) in the pan-EU pilot and present and refine key performance indicators (KPIs) to qualify and quantify the flow of activities, subject perception, cost, quality and compliance.
Deliverables |
Timeline |
Status |
|---|---|---|
| D3.1 Report on decisions and rationale for these decisions on pivotal choices regarding pan-EU pilot (TA(s), N of arms, intervention, countries, including rationale for choices made |
M20 | Completed (confidential) |
| D3.2 Finalised list of KPI’s to be used to qualify and quantify the flow of activities in the pilot |
M20 | Completed |
| D3.3 Draft protocol for pan-EU pilot to be used for small feasibility (dry-run) study ready and aligned across countries |
M22 | Completed (confidential) |
| D3.4 Report on small feasibility study & proposed adaptations to the protocol to be made based on results & final protocol(s) for pan-EU pilot |
M37 | Completed (confidential) |
| D3.5 First study subject approvals package |
M41 | Completed |
| D3.6 Midterm recruitment report |
M50 | Completed |
| D3.7 Audit report on process and ICH GCP deviations for the pan-EU pilot |
M56 | Completed (confidential) |
| D3.8 Report on final evaluation of the pan-EU pilot regarding KPI and learnings |
M68 | Not started |
| D3.9 Report on status of posting results |
M68 | Not started |
| D3.10 Ethics D2, H. Requirement No 2: pre-feasibility information package ready |
M37 | Completed (confidential) |
| D3.11 Ethics D3, HCT – Requirement: human cells/tissues information package |
M41 | Completed (confidential) |
| D3.12 Ethics D5, NEC – Requirement: non-EU activities |
M41 | Ongoing |
| D3.13 Ethics D6 – H – requirement No. 6 |
M41 | Completed (confidential) |
4. Ethical, regulAtory, Gcp and LEgal aspects
WP4 EAGLE had the following objectives:
- Map and analyse the ethical, regulatory, GCP and legal aspects (regulations and guidelines) of the current EU environment for DCT
- Identify challenges and solutions for the evolution of DCT within the clinical regulatory framework, including digital health.
- Analyse the acceptability of the DCT approach for benefit-risk decision making by regulators and HTA bodies.
- Develop an innovative proposal to create a responsible and sustainable ethical and regulatory ecosystem for DCT.
Deliverables |
Timeline |
Status |
|---|---|---|
| D4.1 Map of the EU legislation on DCTs including legal, regulatory, ethical and stakeholder recommendations for conduct of the pan-EU pilot |
M18 | Completed |
| D4.2 SWOT analysis of ethical, legal and operational barriers and enablers for DCT in the EU |
M48 | Completed |
| D4.3 Overview of technical and regulatory implications of DCTs for efficient regulatory decision-making |
M48 | Completed |
| D4.4 Overview of innovative scenarios for a responsible and sustainable DCT ecosystem |
M48 | Completed |
| D4.5 Final EAGLE report |
M68 | Completed |
5. Communication, dissemination & stakeholder engagement
WP5 CODE aimed to:
- To communicate to external and internal stakeholders the key results of the project, and develop a broad consensus on how DCTs should be developed and implemented in the EU.
- To identify stakeholders’ perspectives on the barriers and enablers in DCTs (patients, regulatory agencies, ethics committees, principal investigators, study coordinators, hospitals, pharmaceutical companies) and communicate these perspectives both externally and internally.
- To measure and quantify the paradigm change from traditional trial approaches to induced changes in DCTs from the perspectives of patients and HCPs.
- To assess and tailor the related services of the ‘technology package’ resulting from WP2 TECH for the communication activities.
- To develop a check-list on best practices for setting-up a DCT in EU in consultation with the External Stakeholder Platform (ESP) and internal stakeholders, including a digital HCP researcher certification programme.
- To develop training kits for deploying pan-EU DCTs for principal investigators, HCPs, patients, inspectors, pharmaceutical companies, clinical research organisations; provide a platform for their effective dissemination and promotion; create informational materials to clarify DCTs for prospective trial participants; and develop site support materials that specifically aid in the execution of a DCT.
- To run virtual education/information sessions for company providers/developers of technologies to be deployed for DCTs.
Deliverables |
Timeline |
Status |
|---|---|---|
| D5.1 Communication and Dissemination Plan |
M3 | Completed (confidential) |
| D5.2 Trials@Home website, templates, and social media tools |
M1 | Completed |
| D5.3 Report on the mapping of paradigm changes in the relationships between HCPs and patients |
M41 | Completed |
| D5.4 Report on changing stakeholders’ roles and responsibilities and proposals from stakeholders to overcome any challenges |
M41 | Completed |
| D5.5 Education & Training Sessions |
M71 | Completed |
| D5.6 Set of tools for DCT including training materials for stakeholders (e.g., principal investigators, patients, regulatory representatives and inspectors, etc.) |
M71 | Completed |
6. PROject Management and Synthesis
WP6 PROMS had the following objectives:
- Provide effective project management to all workpackages facilitating timely delivery of all actitivities, within budget and in compliance with the EC Grant Agreement and Consortium Agreement
- Set-up an effective internal communication infrastructure and foster the integrative process within the consortium
- Set-up and manage the External Stakeholder Platform, including liaising with the other WPs and set-up and coordination of the required ESP meetings (operationally, content development within the WPs)
- Plan for, organise and coordinate an open call for additional technology partners
- Develop a sustainability plan for optimal translation and implementation of project results within and beyond the project duration
- Prepare, coordinate and develop an integrated set of recommendations, draft EU guidelines and (educational) tools, synthesized from the outcomes of WP1-5 to facilitate DCT implementation in Europe
Deliverables |
Timeline |
Status |
|---|---|---|
| D6.1 ESP Terms of Reference |
M2 | Completed (confidential) |
| D6.2 Project management manual and templates |
M6 | Completed (confidential) |
| D6.3 First version of Data management plan |
M6 | Completed |
| D6.4 First version of sustainability plan |
M14 | Completed (confidential) |
| D6.5 Open call for additional technology partners |
M12 | Completed |
| D6.6 Final version of sustainability plan |
M72 | Ongoing |
| D6.7 Integrated set of recommendations, draft EU Guidelines and (educational) tools to facilitate DCT implementation in Europe |
M69 | Not started |
| D6.8 Final Data management plan |
M72 | Not started |
| D6.9 Ethics D1a, GEN-Requirement: Independent Ethics Advisor appointed to participate in the independent external expert panel |
M2 | Completed |
| D6.10 Ethics D1b, GEN-Requirement: report, including ethics section on the review of the proposed technology package for the WP3 pan-EU pilot study |
M18 | Completed (confidential) |
| D6.11 Ethics D4, POPD – Requirement No. 4: Updated data management plan |
M43 | Completed |
| D6.12 Ethics D1c, GEN-Requirement: report, including ethics section on the review of the updated version of the Data Management Plan –specific pan-EU pilot Data |
M48 | Completed (confidential) |
| D6.13 Ethics D1d, GEN-Requirement: report, including ethics section on the review of the feasibility study and protocol for the pan-EU pilot study |
M38 | Completed (confidential) |
