Trials@Home Annual Meeting Report
Utrecht, December 5, 2023
UCB generously hosted us at their headquarters in Brussels for the 4th Annual Meeting for the Trials@Home project on 24 and 25 October 2023. It was an excellent meeting with plenty of engagement from attendees. We reflected on the achievements of the last year. We ended the meeting with high anticipation for the next year of Trials@Home – the best is yet to come!
Tero Laulajainen (Head Global Clinical Science and Operations at UCB) welcomed the team and stressed yet again the importance of decentralised clinical trials (DCTs) in research and more so how DCTs can influence the regulators for the benefit of the patients: “I truly want to thank you all for this transversal collaboration and the important work you do for the benefits of patients.”
Overall progress
Kim Hawkins (Sanofi) and Mira Zuidgeest (UMCU), the overall project leads, expressed how impressed they are by the commitment of the project team members and pointed out that ourfinal deliverables are important to keep in view as we proceed into the fifth year. They were also interested in how the team was feeling (making sure we’reall still in good shape to make it to the project deadline!), with this wordcloud as a result:

General project updates
Julia Kopanz (UMCU) presented her Discreet Choice Experiment on Patient Preferences and highlighted that she’s still looking for participants (people living with Type 2 Diabetes) in the study from the Netherlands, Germany, and Austria.
Netherlands: https://trialsathome.com/dce-homepage-nl/
Germany: https://trialsathome.com/dce-homepage-de/
Austria: https://trialsathome.com/dce-homepage-at/
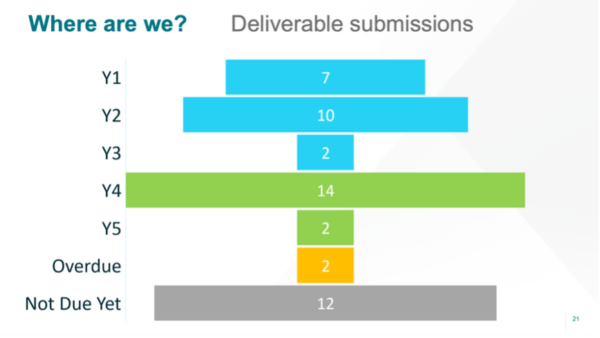
Ashton Babcock (UMCU) presented the state of play in Work Package (WP) PROMS. She gave us an overall update from project management, including an overview on where we are with our deliverables. Seems we’re doing very well, as you can see in in the following image. The project’s deliverables are available on https://trialsathome.com/deliverables/.
She kept juggling with numbers of project results: we have published 11 scientific papers so far, which are all available on https://trialsathome.com/peer-reviews-and-research-outputs/, and more are on the way! Moreover, there have been 34 external presentations in the last year and the number of followers on social media as well as newsletter subscriptions has increased.
The project’s external advisory boards (Scientific Advisory Board (SAB), the External Stakeholder Panel (ESP) and the Patient Expert Panel (PEP) have been consulted many times throughout the project and remain at the project’s disposition for further consultation until the end of the project.
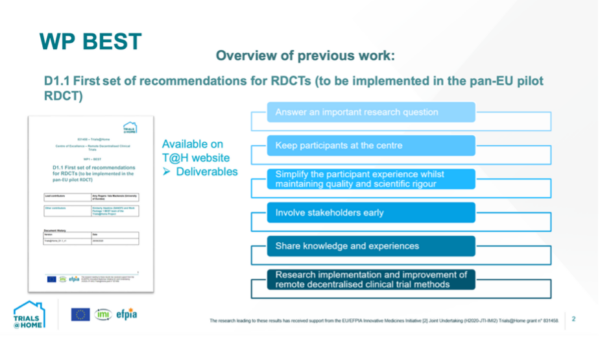
Next up was Isla MacKenzie (University of Dundee) who presented the state of play for WP BEST. She highlighted the findings of their initial scanning of DCT case studies, and pointed out that the findings of the case study scanning can be consulted in various publicly available papers.

Furthermore, Petra Naster (Vital Transformation) and Eduard Vardianu (Labcorp/Fortrea) updated the consortium on what has been realised on the comms side of the project in WP CODE.
RADIAL updates
 The update on the project’s clinical trial RADIAL was presented as a panel discussion with Bart Lagerwaard (UMCU, the sponsor), Paul Bodfish (MRN, representing the Quality working group), Danny van Weelij (Julius Clinical, the CRO), Bobby Davey (eClincial Health, the tech platform provider), Lampros Mpaltadoros (CERTH-I, speaking on behalf of the RADIAL Helpdesk) and Jesper Hallas (Syddansk University – Remote site in Denmark running RADIAL). The panel was moderated by Mira Zuidgeest (UMCU, PI RADIAL).
The update on the project’s clinical trial RADIAL was presented as a panel discussion with Bart Lagerwaard (UMCU, the sponsor), Paul Bodfish (MRN, representing the Quality working group), Danny van Weelij (Julius Clinical, the CRO), Bobby Davey (eClincial Health, the tech platform provider), Lampros Mpaltadoros (CERTH-I, speaking on behalf of the RADIAL Helpdesk) and Jesper Hallas (Syddansk University – Remote site in Denmark running RADIAL). The panel was moderated by Mira Zuidgeest (UMCU, PI RADIAL).
The latest overview of the recruitment status of RADIAL was presented. The panellists reflected on this status and on their own experiences with the trial so far. Lampros shared an update on the RADIAL helpdesk, which uses a customized ticketing system for efficient communication between the CRA, site personnel, and the consortium experts. The learnings from RADIAL, both on the level of data and on the hands-on experience, are captured and will be shared in reports and papers at a later stage. More information about RADIAL here: https://trialsathome.com/about-radial/.
After the panel, Brian Guthrie (Fortrea), the new TECH WP Lead, updated us on the status of the TECH WP. As most of the trial “construction” is done already the main focus of WP TECH is now on the RADIAL helpdesk and knowledge base.
Guest speakers
 Jiajie Zhang (Sanofi) delivered a keynote lecture and educated the team on where we stand with AI in DCTs and how AI can speed up the creation and analysis of trials. He mentioned three main areas where the application of AI is evolving, the first of which is wearable devices. Those devices collect a lot of data, which can be used to train accurate AI models to transform the collected data into meaningful endpoint information.
Jiajie Zhang (Sanofi) delivered a keynote lecture and educated the team on where we stand with AI in DCTs and how AI can speed up the creation and analysis of trials. He mentioned three main areas where the application of AI is evolving, the first of which is wearable devices. Those devices collect a lot of data, which can be used to train accurate AI models to transform the collected data into meaningful endpoint information.
The second area is that of computer vision technologies. Nowadays, many people own a smartphone with a good camera which can be used to take pictures or videos that can be analysed with AI, for example for counting skin lesions. The third area is the use of large language models to give patients a better experience, for example for answering patient’s questions or for patient engagement purposes. It was very interesting and enlightening to hear more about these possibilities.
Colm Carroll (IHI) updated us on the Innovative Health Initiative. He started off by saying how excited he is to join the Trials@Home annual meetings as he always learns more about the complexity of DCTs and the project in general. Colm brought to our attention that the day before the annual meeting, IHI published drafts (subject to change) of future calls that can be found on the IHI website. His main takeaway message for the consortium was to keep mindful of the long-term impact andstrategies to maximise this impact.
At the end of the first day, Joana Claverol (Barcelona Children’s Hospital) presented on their findings of their analysis more than 200 paediatric trials: “Site, patient and investigator vision in the real implementation of DCTs in a paediatric setting”. She concluded that each trial is different and not all paediatric trials will benefit from a digital strategy, so it’s important to ask the “users” if a digital strategy is needed or not.
This led us seamlessly to the panel discussion on paediatric trials at the end of day one. Rick Grobbee (UMCU) welcomed Loic Notelet (Sanofi), Martine Deligner-Kremer (ESP), Theofaneia Tsachalina (PEP), Joana, and Isaac Rodrigues-Chavez (regulatory consultant) to the stage, where they all discussed the options for DCTs in paediatrics. The panel basically seconded the conclusions of Joana’s study and pointed out that in paediatric studies you do not only need to consider the patient but also the family, and that taking away some burden via digitalisation is an option. Involving patients/families in the creation of the trial protocol is therefore highly recommended.
On the second day, Craig Lipset (DTRA, ESP) joined us live from the US to give us an update on what DTRA has achieved so far in the DCT space and what their plans are.
Future lookout
The second day started with the presentation of what the Executive Board (ExBo) has agreed will be the format of the final recommendation of the project. Kim and Mira presented this format, followed by an interactive workshop which led to further agreement on the structure of the recommendations.
Related to the format of the final recommendations, Petra Naster (WP CODE Lead), presented the outcomes of the brainstorm session on the website overhaul we had at the Semi-Annual Meeting six months ago. When the final recommendations format is carved in stone, the website homepage and underlying pages will be reworked aswell to match the structure.
Also highlighted was the new WP CODE working group named the “Layman Team”. This effort, led by CODE and the patient experts (PEP members), will translate (not summarise) existing outputs from theproject such as papers or posters that are of immediate interest to patients into layman language.
Annemarijn Douwes (UMCU, PROMS) presented the sustainability plans for the project, another important aspect of the project as we want the impact of our work to last long after the project ends. Outputs were determined and potential partners were highlighted.
After lunch on day two, we had one final session before we all headed home again: a scientific inspiration session lead by Tessa van Rijssel (UMCU), Julia Kopanz (UMCU) and Rachel Copland (University of Dundee). The title of the session was “A Reflection on DCT Case Studies” where they presented a few case studies (fictitious or not). In small groups, we discussed several aspects of DCTs to come up with (out-of-the-box) recommendations on these topics on how to best conduct a DCT.
As usual, the meeting came with many opportunities for questions and comments from all who joined both in person and virtually, as well as networking opportunities throughout the meeting.
 The meeting closed with a big thank you from Susanne Dorn (UCB) and the project leads. The next day, we launched a feedback survey to find out if the rest of the group enjoyed the meeting as much as we did. The 31 people who responded seemed to think so, with overwhelmingly positive responses to how useful and interesting the content was, and even some suggestions for the next project meeting.
The meeting closed with a big thank you from Susanne Dorn (UCB) and the project leads. The next day, we launched a feedback survey to find out if the rest of the group enjoyed the meeting as much as we did. The 31 people who responded seemed to think so, with overwhelmingly positive responses to how useful and interesting the content was, and even some suggestions for the next project meeting.
We look forward to telling you all about the Semi-Annual Meeting in 6 months!



This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 831458. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Notes for editors – not for publication
If you would like more information, please contact the spokesperson at the UMC Utrecht.
Joris Prinssen: +31 6 2571 0234
press@umcutrecht.nl