About RADIAL, the Trials@Home proof-of-concept study

The RADIAL trial is testing out whether future clinical trials can be successfully run from participants’ homes rather than hospitals or clinics – and the best way to do this.
The trial is open to people living with type 2 diabetes who are on long-acting basal insulin.

About the RADIAL trial
RADIAL is different from most clinical trials because it is designed to test out a way of running future trials.
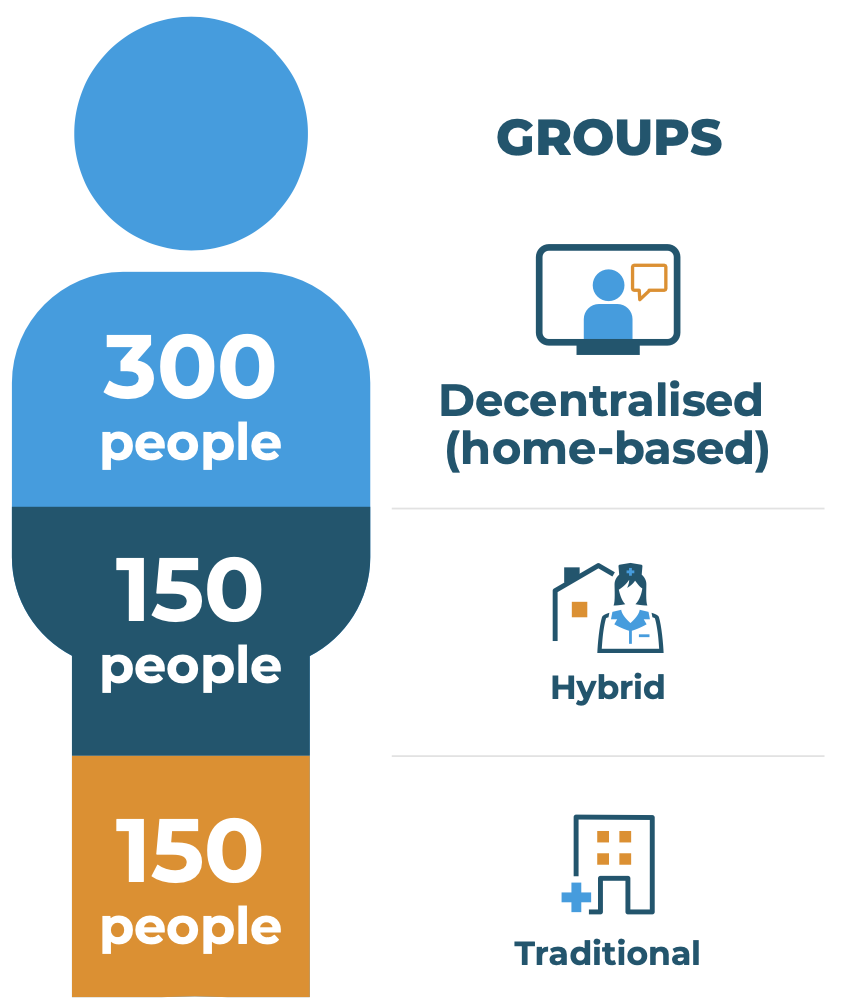
The aim of RADIAL is to directly compare a decentralised (home-based) approach with both a standard, traditional (site-based) and a hybrid approach. To make this comparison, Trials@Home considers that an ideal focus for the RADIAL trial is people living with type 2 diabetes who are on long-acting basal insulin. Importantly, people living with type 2 diabetes were closely consulted on the design of the trial and on the way information about the trial is explained.
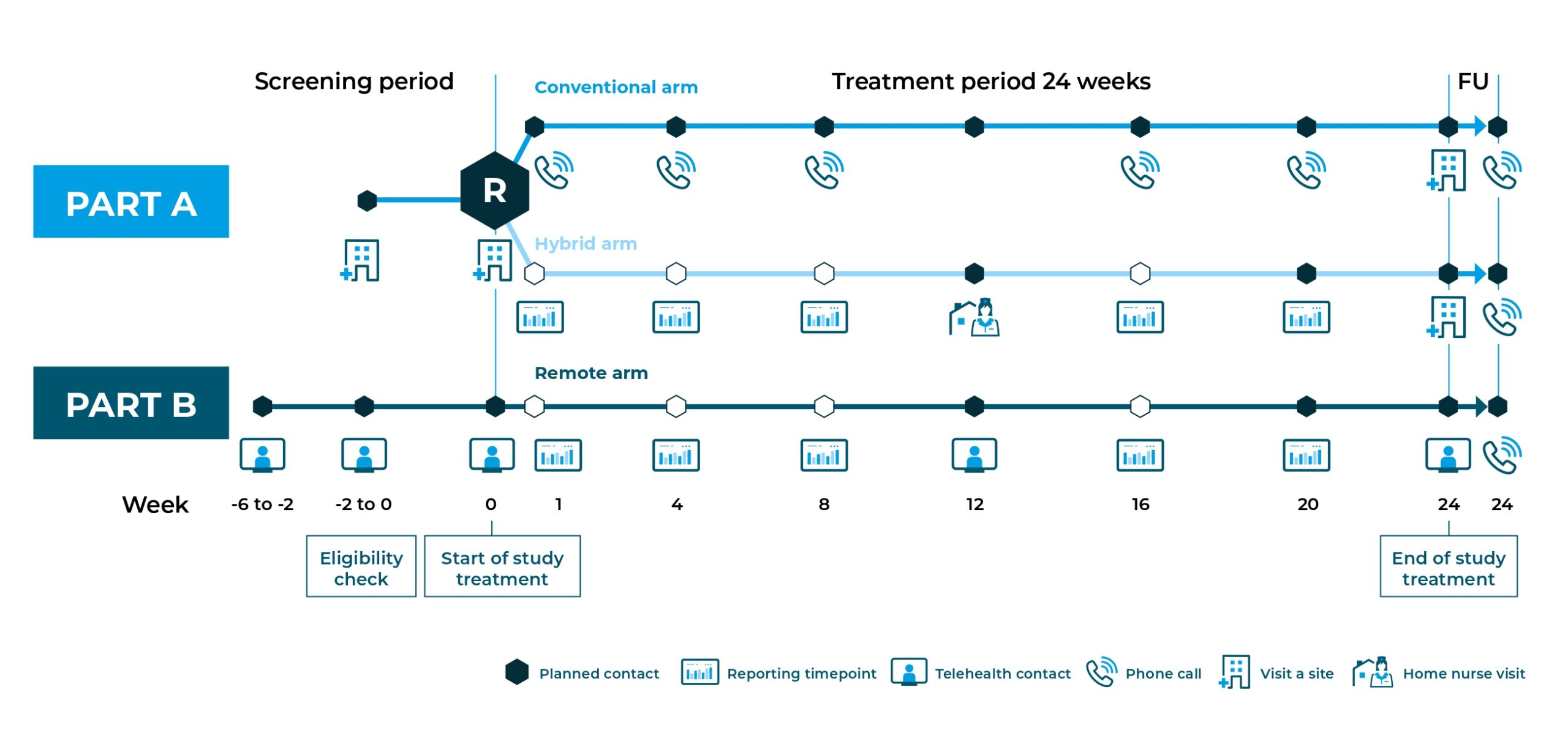
The trial will be conducted in three arms as shown on the diagram. Participants will be recruited in the traditional fashion for Part A and will be randomised in the conventional arm or the hybrid arm. Participants for Part B will be recruited fully remotely.
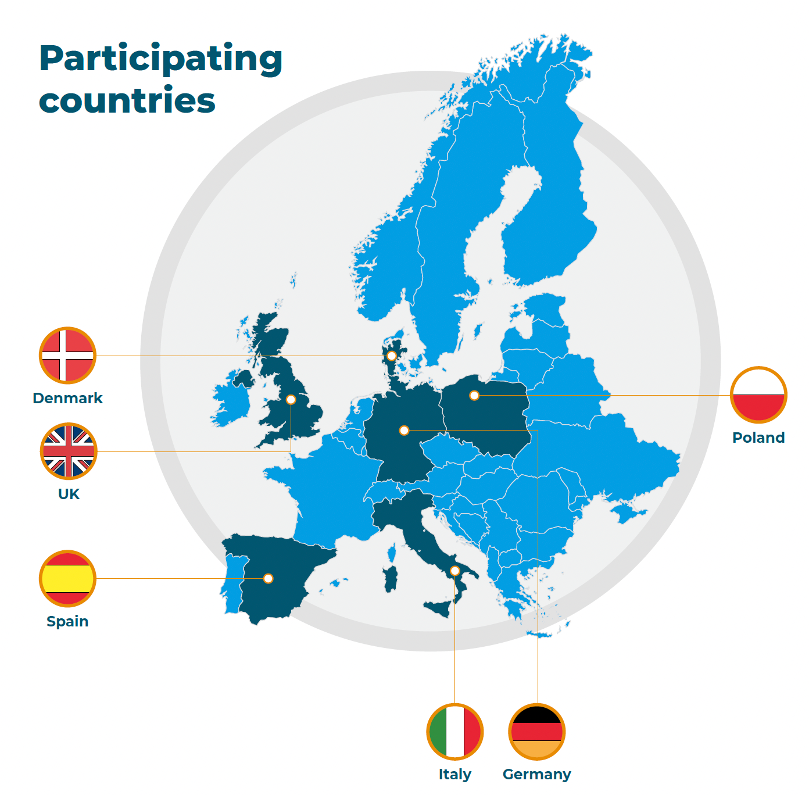
Participating countries
RADIAL will be conducted in five European countries and the UK. How we selected these countries is documented here: RADIAL Country selection process.
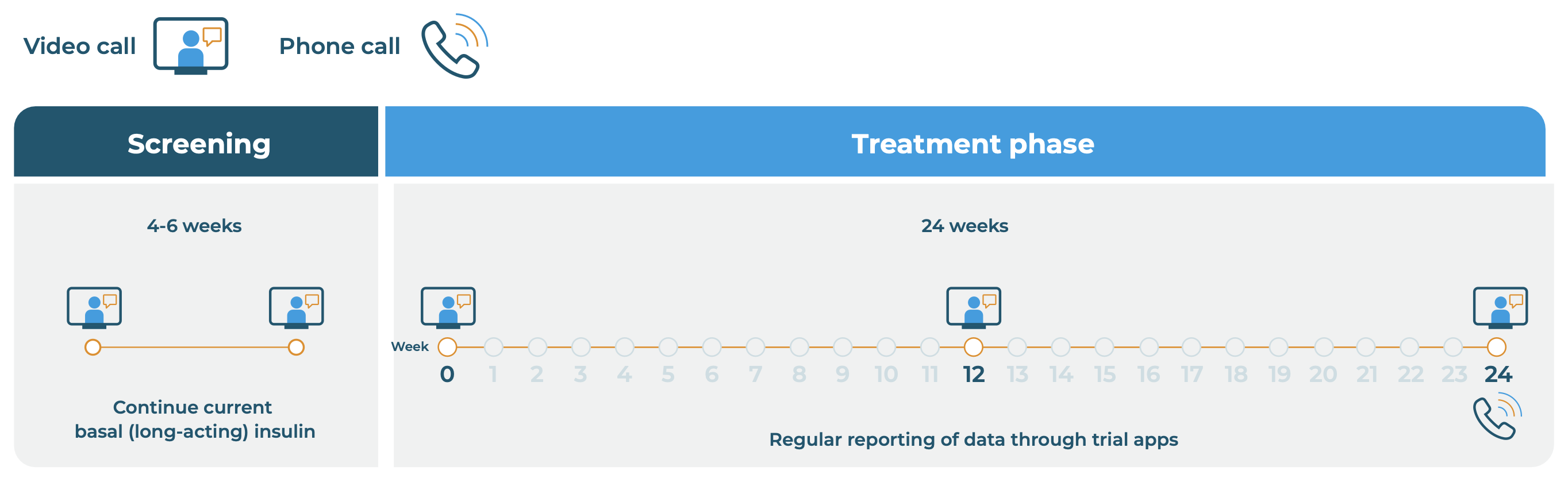
RADIAL trial timeline for remote participants and participant numbers
The RADIAL trial will last for about 7½ months. The first six weeks will be the screening phase where the research team will make certain the trial is suitable for participants who apply. The main treatment period of the trial will last for 6 months (24 weeks), with three scheduled video calls with the trial doctor.
What are we researching in RADIAL?
This study has two primary objectives. First one is to assess potential benefits of a decentralised clinical trial approach on participant retention, recruitment, diversity, cost, site staff and participant satisfaction.
Second one is to determine acceptability of a decentralised clinical trial approach regarding data quality, safety oversight and medication compliance within the arms that have a different degree of decentralisation.
The following objective is related to the clinical endpoints and is a secondary objective for this study to determine whether the efficacy of an intervention with insulin Toujeo® is within the accepted range within the arms with different degrees of decentralisation.
Do you need more info about RADIAL or do you want to join the trial?
Useful links
- RADIAL Launch Webinar: Trials@Home proof-of-concept study RADIAL is ready for patient recruitment
- RADIAL Press Release: Study to test innovative decentralised clinical trial model is ready for patient recruitment.
- RADIAL CTIS application – search for RADIAL or via the EUCT number: 2022-500449-26-00