First set of recommendations for RDCTs (D1.1)
Lead contributors: Amy Rogers / Isla Mackenzie (University of Dundee)
August 26, 2020
Clinical trials are a crucial step in the development and testing of medical treatments. They are essential to ensure that new treatments are safe and effective.
Traditionally, clinical trials have taken place in hospitals or other research sites, often requiring participants to attend several face-to-face study visits. While these trials produce results, they can do so at a great burden to participants and risk excluding people who are unable or unwilling to travel to study visits.
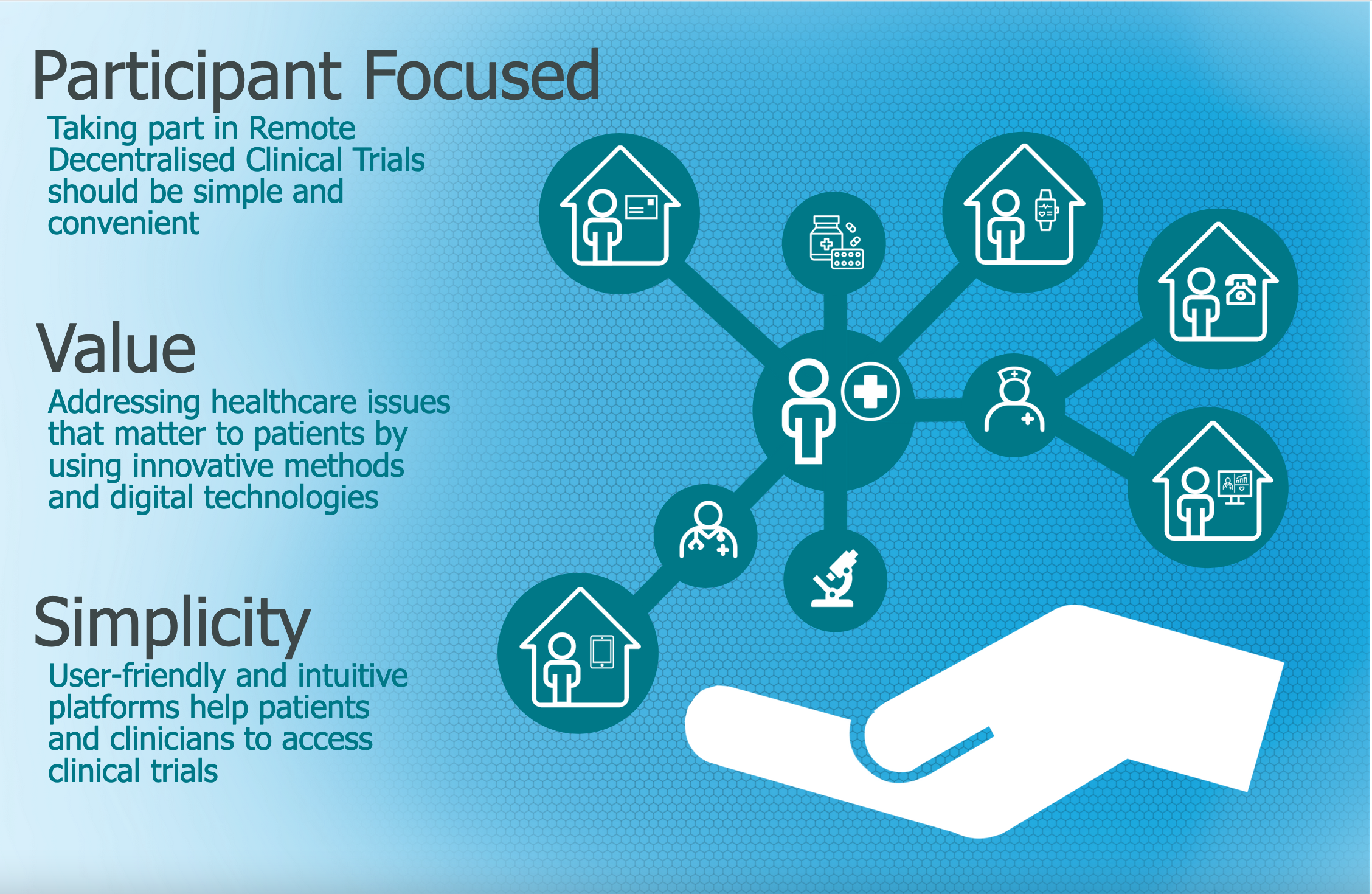
Remote decentralised clinical trials (RDCTs) are one way to make trials more accessible. RDCTs are centred around participants. Using technology can allow people to take part in clinical trials in their own home with no need to travel to attend study visits or to take substantial time off work or away from family. RDCTs have the potential to make taking part in a clinical trial simple and convenient.
These draft recommendations are based on in-depth research, conducted over a 12-month period, into remote decentralised clinical trial methods. They apply to all aspects of RDCTs from design, planning and set-up to close-out and reporting.
3 Key Recommendations
- Answer an important research question
- Keep the focus on participants
- Simplify the participant experience whilst maintaining quality and scientific rigour
Full recommendations will be published at the end of the IMI Trials@Home project in 2024.
