Training Materials

In order to support future implementation of DCTs in Europe we will design online training and education to introduce the concept of DCTs, how to design and run them and which technologies are most suitable. These trainings will also target patients and patient organisations in order to make them familiar with the concept of DCTs allowing for co-creation of the trial protocol and efficient patient recruitment and retention for the DCT proof-of-concept study within Trials@Home, as well as beyond the project.
What is IMI? And what is Trials@Home?
We have created some short introductory videos on IMI and Trials@Home as a project.
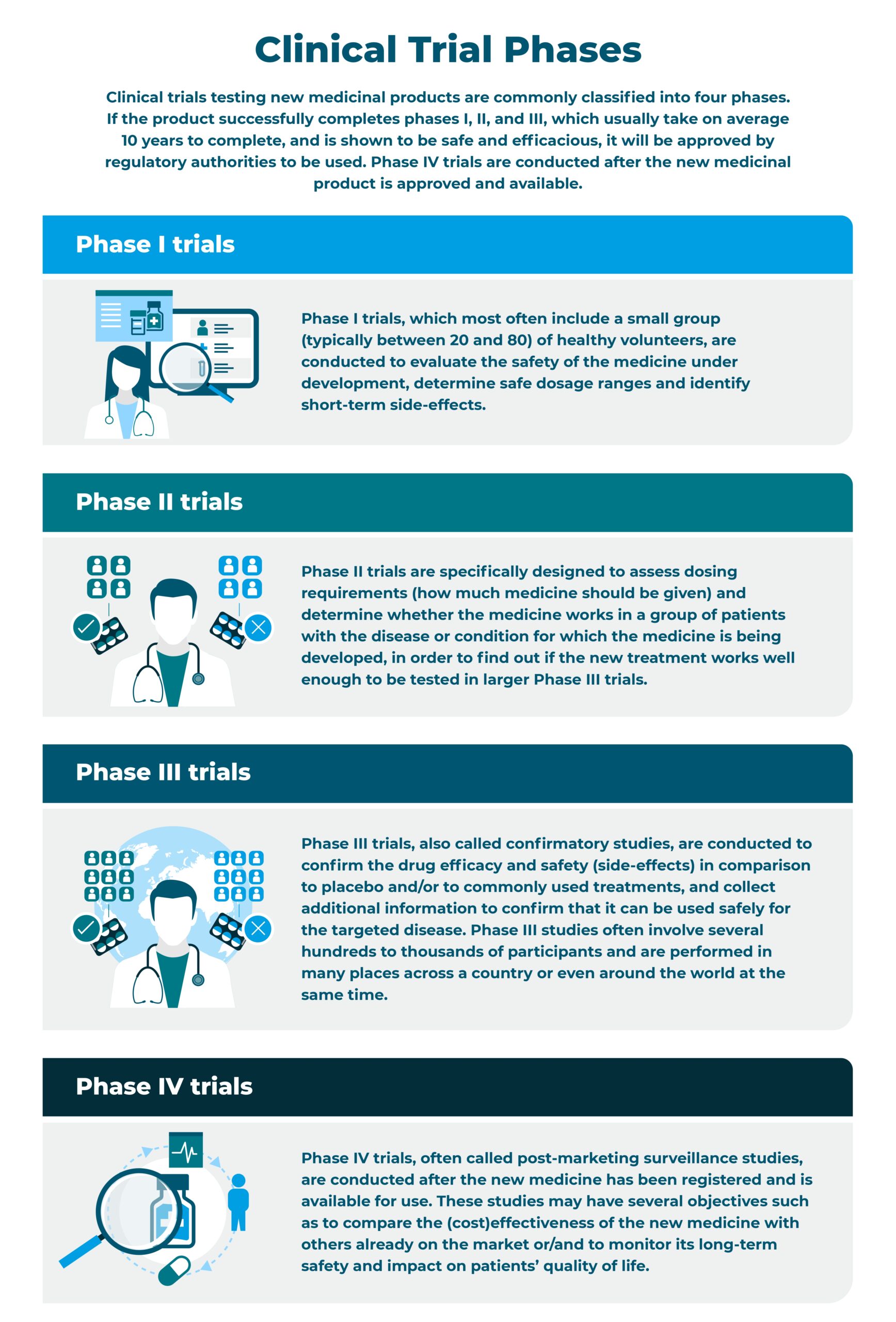
What are clinical trials and which different types exist?
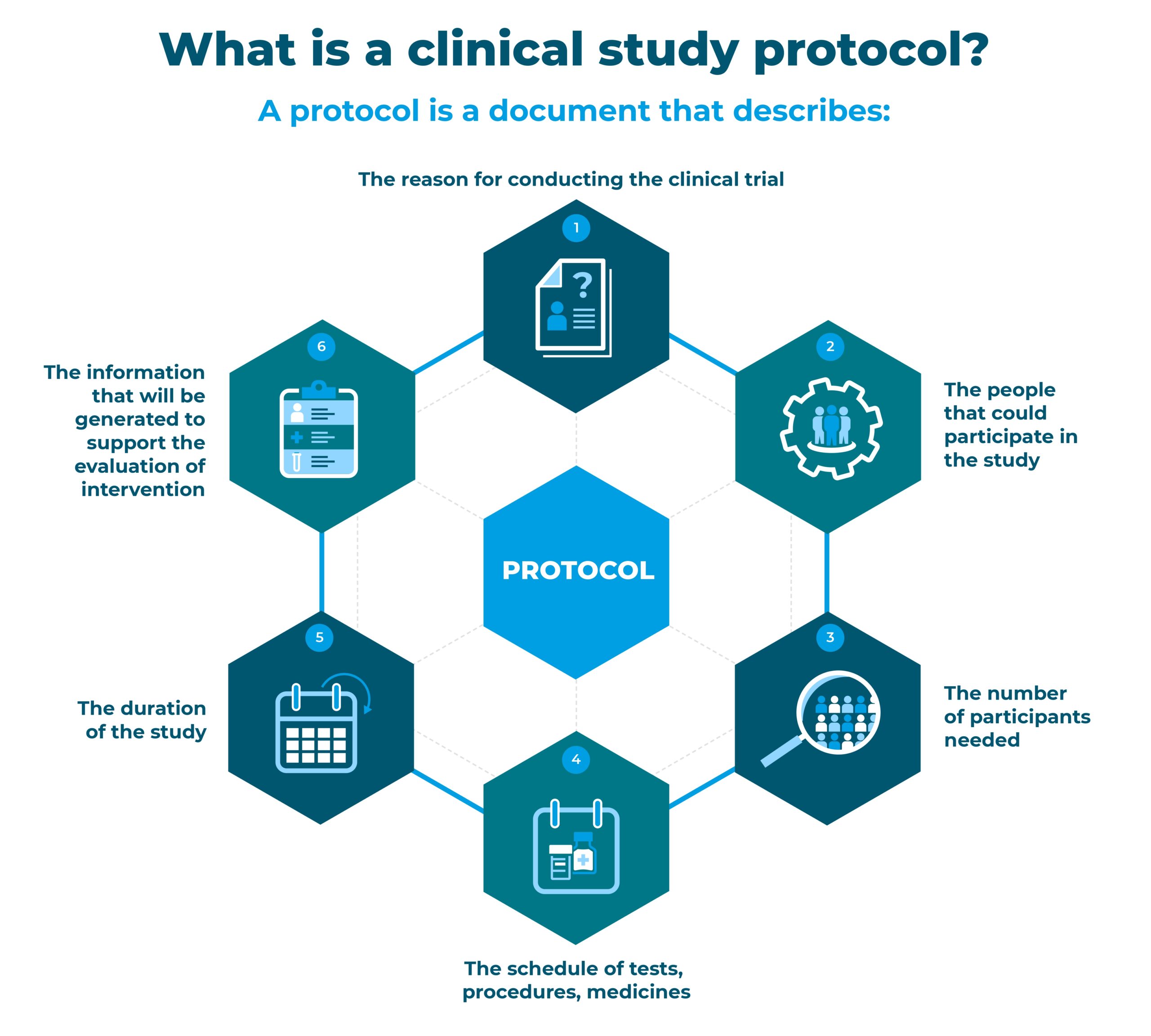
The sponsor is the organization which takes responsibility for the initiation, management, and/or financing of a clinical trial. Any trial must be conducted according to international and regional ethical principles that have their origin in the Declaration of Helsinki, and to scientific, regulatory and quality standards consistent with international Good Clinical Practices. People can participate in the study only if they have been fully informed about how it will be set up and run, what its benefits and potential risks are, and after having signed an informed consent form.
It is important to highlight that for the sake of transparency, and as per European Regulation, sponsors must disseminate and share a summary of the results of their trials with the trial participants and the public at large (two summaries must be released, one more targeted to researchers and another in lay language for the general public). With the implementation of the European Clinical Trial Regulation, these summaries will be available through the European Medicines Agency Clinical Trial Information System (CTIS) released on January 31, 2022.
What are decentralised clinical trials?
Interested in more training? Check out our research videos
Research papers explained
Research Papers Explained – first paper translated!
Experiences of clinical trials in homes and local settings outside of clinics - a research project using interviews Utrecht, June 25, 2024 Research Papers Explained – first paper translated! [...]

