Events

Events & Speaking Engagements
CLOSING EVENT :: Trials@Home, a home run?
Recommendations after leading the way on DCTs in Europe
SCOPE: To share learnings from Trials@Home and look to the future of decentralised clinical trials
LOCATION: The Railway Museum Utrecht, The Netherlands; or online
DATE: 31 October, 2025 09.00 – 17.30 CET
Info & Registration
15th Annual Outsourcing in Clinical Trials Europe 2025
29-30 April, Barcelona, Spain
Cameron Keighron (IDF) will be running a round table discussion:
|
How minimising participant burden leads to on-time enrollment and completion
|
but you can also find Cameron and the IDF team here:
-
30 minute lecture on Day 1 in the Patient Centricity Theme
-
Panel Discussion on Day 1 around barriers to participation in clinical trials
-
Two round tables on Day 2 on exploring the importance of lived experience in research
More info here: https://www.arena-international.com/event/octeurope/#agenda
CCRA Professional Awareness Update Seminar
17 December 2024 | London, UK
Paul Bodfish (MrN) will be presenting a RADIAL update at the meeting on 17th December in London. CCRA is the Clinical Contract Research Association of which MRN and most CROs in the UK are members.
More info here: https://www.ccra.org.uk/meetings/20241217/Programme.asp
ISPOR Europe 2024
17-20 November 2024 | Barcelona, Spain
On Monday 18 November, Julia Kopanz (UMCU) will present her poster "Do People Prefer to Participate in a Clinical Trial From Home? A Discrete Choice Experiment in Persons Living With Type 2 Diabetes Mellitus" with the interim results of her research during poster session 2.
More info here: https://www.ispor.org/heor-resources/presentations-database/presentation/euro2024-4014/144963
CLINICAL TRIALS SYMPOSIUM, 1 day hybrid event Bucharest Onsite & Online
8th of November, 2024
Assistant Prof.Bart Lagerwaard, University Medical Center Utrecht will present on:
Initial learnings on patient centricity from RADIAL, a pan-European, decentralised and hybrid proof-of-concept trial
Full programme and registration info here
ICTMC 2024, Edinburgh, Scotland
30 Sept – 3 Oct 2024
Julia Kopanz was present on behalf of Trials@Home and participate in the following session:
Oral presentation: Physical examination in phase 3 and 4 drug interventional type 2 DM clinical trial protocols – what is to be done and why?
Full programme and registration info here
WEON 2024 -EPIDEMIOLOGY & PLANETARY HEALTH
May 29-30, 2024
The Trials@Home consortium partners were well represented at this conference.
Rick Grobbee delivered a keynote presentation: Epidemiology 3.0
Mira Zuidgeest co-presented a masterclass: Innovation in Clinical trials – Why should we innovate and how?
Amos de Jong presented on: Does conducting clinical trials at home improve participant representativeness?
And Julia Kopanz presented on two occasions: Physical examination in phase 3 and 4 drug interventional type 2 diabetes mellitus clinical trial protocols – what is to be done and why? and What drives the decision to participate in a clinical trial from home? – a focus group study in persons living with type 2 diabetes mellitus?
DELIBERATING ETHICS IN TRIALS – SCOPE AND NECESSITY OF ETHICAL OVERSIGHT
2nd May 2024, 13:00 – 17:00 (UTC+1)
On May 2nd, Dr Amy Rogers participated in this online workshop where she presented on "Ethical considerations for Decentralised Clinical Trials"
Link to the video of her presentation here
DIA Europe 2024, 12-14 March, Brussels.
On Wednesday, 13 March 2024, 15:10 – 16:20 CET, Dimitrios Athanasiou, our World Duchenne Organisation project partner, will be a panelist on "Clinical Trials with Decentralised Elements" (S0202, Track: Clinical Trials Development & Operations)
The session is chaired by Cecile Ollivier, Critical Path Institute, Netherlands and Monique Al, Central Committee on Research Involving Human Subjects (CCMO), Netherlands
Session Description:
This session will discuss the evolving global landscape for decentralised clinical trials, to (1) impact of recent and ongoing DCT initiatives and activities by various stakeholders (2) evaluate where we are in fulfilling the promise of DCTs for each stakeholder (3) debate what still needs to be done to advance the use of decentralised elements.
And on Thursday, March 14th, 09:00 – 10:10 CET, Solange Rohou, AstraZeneca, will be a panelist on "Drug Repurposing: Opportunities & Challenges" (Track:10 Regulatory Strategy and EU Pharmaceutical Policy)
More info here: https://www.diaglobal.org/en/flagship/dia-europe-2024
Insights in the Medical Science City of Graz, February 29, 2024, Medical University of Graz
This meeting is hosted by Human.technology Styria and Sten Hanke, WP2 lead, will represent Trials@Home.
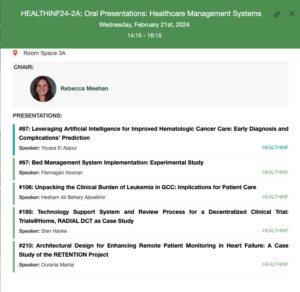
HEALTHINF 2024 – 21_23 February, Rome, Italy.
HEALTHINF, the 17th International Conference on Health Informatics, is part of BIOSTEC, the 17th International Joint Conference on Biomedical Engineering Systems and Technologies.
Registration to HEALTHINF allows free access to all other BIOSTEC conferences. More info on the programme and registration here.
Sten Hanke, WP2 lead, will present on Trials@Home in the following session:
EUCROF24 – Prague – 19-20 February.
EUCROF is delighted to announce that Bart Lagerwaard from UMC Utrecht will be presenting the session "Do Decentralised Clinical Trials improve representativeness?" at EUCROF24.
Bart said "Decentralised clinical trials (DCTs) move the trial activities to the patient's home and have the potential to enhance the conduct of clinical trials in multiple ways, including increasing the accessibility to the trial for a wider population by eliminating geographical barriers, and at the same time offering the participants the comfort and convenience of performing the trial from their homes. Does this potential of DCTs improve the representativeness of the trial?"
In this presentation Bart will share initial learnings from setting-up and conducting the RADIAL trial, a decentralised and hybrid proof-of-concept trial that aims to answer these (and many other) research questions on DCTs. The session will highlight the interconnections between operational trial innovations and considerations for representativeness of the trial.
Find out more and view the full programme here: https://lnkd.in/eQP39Kx3

Diabetes Professional Care, 15 November, 2023
London
At 14.15 CET Bart Lagerwaard will speak at the Diabetes Professional Care Conference in London https://lnkd.in/ens5jCTN about Bringing clinical research to the homes of people living with Type II diabetes – The future of clinical research?
He will present an overview of the project and RADIAL, our proof-of-concept study that will also run in the UK.
Clinical Trials Europe,29 – 30 November, 2023
CCIB, Barcelona
*IN-PERSON EVENT ONLY*
Trials@Home is proud to be a Media Partner for Clinical Trials Europe.
Trials@Home will also be featured at the conference in two different sessions.
Panel Discussion, with Stuart Redding, CEO, MRN
Wednesday, 29 November 2023 12:00 – 12:45 CET/CEST (Cent Europe Summer, GMT+2)
Alternative Site Models – Innovation to Traditional Methods
- Mobile sites
- Pharmacies
- Point of Care to mobilise entire healthcare systems
- Pop up sites- open on demand based on community needs
- How can these different models work? Benefits and Limitations
- What framework is required to consider implementation of these models?
- How do companies ensure quality and oversight when looking at alternative models?
- How efficient are these new models?
Presentation by Paul Bodfish, Consultant, MRN
Thursday, 30 November 2023 11:30 – 12:00 CET/CEST (Cent Europe Summer, GMT+2)
Decentralised Trails Designed for Chronic Conditions
- What will trials for chronic conditions look like moving forward?
- Can these be fully managed outside of a hospital and what could this look like?
- Case study: Radial trials
BIO 2023, 5-8 June 2023, Boston, US
Join us at #BIO2023! Craig Lipset will be moderating a Panel around Meta-Collaboration to Improve Decentralized Trial Excellence.
Tuesday, June 6 | 11:00 – 12:00 PM ET | Room 254A
The panelists will share updates on the scope of their work in the field as well as where collaboration opportunities exist across the ecosystem. Hear perspectives from Kim Hawkins (Sanofi & IMI Trials@Home), Morgan Hanger (Clinical Trials Transformation Initiative (CTTI), Karen Noonan (Association of Clinical Research Organizations), and AMIR KALALI, MD (Decentralized Trials & Research Alliance (DTRA)

IMI impact on clinical trials – 25 May 2023, 14.00-15.30 CET
This online event will showcase how the Innovative Medicines Initiative (IMI) projects are delivering results that will improve how clinical trials are run.
Clinical trials are essential to demonstrate the benefits and risks of new interventions such as medicines and vaccines. However, conducting clinical trials can be challenging due to factors such as slow participant recruitment, low participant retention, limited availability of trained investigators and high quality clinical sites to run clinical trials, the burden of trial-related visits to the clinical site, high costs, limited generalisability of trial results to routine clinical practice, high costs, etc.
Collaborations among stakeholders such as academia, industry, patients, hospitals/sites and regulators are critical to address these challenges and drive the modernisation of clinical trial tools and methodologies so that trials better address patients' needs.
A number of Innovative Medicines Initiative (IMI) projects were set up with the goal of optimising certain features of clinical trials, ranging from operational aspects to trial methodology, and ensuring trials have a patient- and caregiver-centred approach.
This event is an opportunity to feature some of the IMI project results that will contribute to the Accelerating Clinical Trials in the EU (ACT EU) initiative launched by the European Commission, the Heads of Medicines Agencies and the European Medicines Agency.
Mira Zuidgeest will present the Trials@Home project during this session.
The recorded session + presentations are now available: https://www.ihi.europa.eu/news-events/events/imi-impact-clinical-trials
International Clinical Trials Day (ICTD) 2023, 23 May 2023, Warsaw and online
Decentralised Clinical Trials: challenges and opportunities
ECRIN together with our Polish National Partner, PolCRIN (Agencja Badań Medycznych) look forward to welcoming you to ICTD 2023 in Warsaw. This year, the discussions will focus on Decentralised Clinical Trials: challenges and opportunities.
We have an outstanding lineup of experts joining us to share their perspectives and experiences with decentralised clinical trials.
Rachel Copland (Dundee) will provide the keynote address, which will share lessons learned from the IMI Trials@Home project about decentralised clinical trials in Europe
Decentralized Clinical Trials 18-20 April, Boston, USA
UNRAVEL THE HYPE, LEVERAGE THE BENEFITS AND EFFICIENTLY RUN YOUR NEXT CLINICAL TRIAL WITH CONFIDENCE
In Collaboration with Tufts Center for the Study of Drug Development – Hear the Latest Trends Impacting Clinical Trial Research
On Amy Rogers (University of Dundee) will participate in this conference on two occasions:
- 18 April: 11:40pm – 12.20pm EST: PANEL: Collaboration, Transparency and Working Together Across Different Consortiums and Associations
- 19 April: 2.20pm – 2.50pm EST: Trials@Home – An Update from the European DCT Perspective
GetReal Institute 1st Annual Meeting, 16 March, Utrecht, The Netherlands
"A European Outlook on Real World Evidence in a Global Context"
The conference will discuss RWE developments in a global context, reflecting upon progress in Europe, North America, Latin America, and Asia Pacific.
The aim of the conference, and the GetReal Institute, is to inform the RWE community of recent developments and the future direction of RWE, highlighting emerging initiatives and ongoing challenges, but more importantly the opportunities to collaborate and collectively address these challenges.
During the abstract presentations, Mira Zuidgeest (UMCU) will present the Trials@Home project in the "Creating and Curating RWE evidence" stream.
35th Workshop of the EURORDIS Round Table of Companies (ERTC)
"Bringing Clinical Trials into the Future"
Wednesday, 22nd February 2023 – Doubletree by Hilton Brussels City in Brussels, 09:30-17:00 CET
EURORDIS-Rare Diseases Europe, along with the broader community of patient organisations, have been on a journey to shape the European policy landscape to bring more and better treatments for rare disease patients. From advocating for the EU Regulation on orphan medicinal products (1997) to the EU Directive on Patients' Right to Cross-Border Healthcare (2011), the rare disease community has continued to lead collective efforts towards the adoption of legislations impacting lives.
Another case in point is the evolution of the Clinical Trials Regulation, which will profoundly change the way trials are conducted in Europe and improve further healthcare delivery pathways for rare disease patients. This workshop is set to evaluate the potential and ambition of this legislation and its implementation, one year after it came into effect in Europe, and to appreciate the benefits and challenges of this new approach to clinical practice for developers, patients and regulators.
Sonia Houston-Pichardo (BI) will present on how our Patient Expert Panel (PEP) was involved in the creation process of RADIAL and on the technology our proof-of-concepot study will use.
More info and registration here: https://www.eurordis.org/fr/publications/35th-workshop-of-the-eurordis-round-table-of-companies-ertc/
Enhancing patient-centric outcome measures and clinical trials with Digital Health Technologies, 12-13 December 2022 (online)
Building on the great momentum in Europe, with initiatives such as ACT-EU or the EMA Regulatory Science Research needs, and advances in IMI projects such as IDEA-FAST or Mobilise-D, EFPIA is organising a multistakeholder workshop on the use of Digital Health Technologies (DHTs) in drug development, with a focus on digital data including digital endpoints. This workshop will offer an opportunity to discuss how to optimise DHTs development, evidence generation for validation/qualification, and regulatory pathways, and to clarify some of the challenges faced so far.
To be held virtually on 8-9 November 2022, the workshop seeks to build a roadmap for enabling the use of DHTs in drug development in Europe and beyond. It will be designed with plenary sessions and interactive breakout sessions to integrate views and expectations of the various stakeholders, and to share learnings and solutions. Discover our draft agenda here.
This event is free to join. Register now to join the conversation.
ISPOR Europe 2022: Collaborating Across Borders: Building & Using Evidence to Enable Access, 6-9 November, Vienna, Austria and Virtual
On 7 November, 10:45-11:00 AM, Julia Kopanz (UMCU) will present on: Patient Preferences on Decentralization of Clinical Trials: Identifying Attributes in a Focus Group Study
Also on Monday 7 November, Julia will present the same paper from 12:30 to 13:15 pm CEST in the Student Poster Tour. You can find the poster here.
Clinical Trials Europe: Step Into The New Era Of Clinical Trials: Future-Proof Your Strategies To Ensure Clinical Trial Success, 7-9 November, RAI, Amsterdam
On 8 November at 14h00 CET, Mira Zuidgeest (UMCU) will present on: Decentralized Clinical Trials – Initial Learnings from Trials@Home and the RADIAL Study
- During this session we will discuss the topic of Decentralised Clinical Trial (DCT) approaches from several angles.
- What are the (perceived) opportunities and challenges of DCTs from different stakeholder groups?
- What can we learn from case studies with decentralised approaches?
- How do we intend to provide insight into some of these challenges and opportunities through the proof-of-concept-study RADIAL (a methodological study comparing decentralized approaches to conventional trial approaches regarding several KPI)
- What are the initial learnings from the submission and start-up of the RADIAL study
- Lastly, we will introduce ways forward for DCTs, as a start of for a Q&A
This in-person event is free to join. Register now to join the conversation.
DIA Clinical Trials and Data Science Conference, 19-20 October, Amsterdam
On 20 October at 11am CET, Mira Zuidgeest (UMCU) and Amos de Jong (UU) will be speaking in break out session 1A: Decentralised Clinical Trials on "Learnings from IMI Trials@Home".
Later on in the day, at 4pm CET, Solange Corriol-Rohou will join the plenary session 2 "Patients as Partners – What's in it for the Patient?".
More info on how to register and the full programme can be found here
EMA ACT EU multi-stakeholder meeting on decentralised clinical trials, 4 October, Amsterdam (online)
The Accelerating Clinical Trials in the EU (ACT EU) programme is hosting a multi-stakeholder workshop on decentralised clinical trials (DCTs) on behalf of the EU DCT project, bringing together participants from all areas of the research community to share perspectives on this type of clinical trials.
During the workshop, the EU DCT project group will present the work of the European medicines regulatory network on decentralised clinical trials collaboration, including the planned publication of a recommendation paper on the use of decentralised elements in clinical trials in the fourth quarter of 2022.
The onsite workshop is open to invited participants only, but the workshop's plenary sessions will be broadcast live on this website. Any interested party can follow this broadcast. A video recording of the plenary session is made available after the event. Processing and publication of the video recording typically take up to 60 days. The agenda of the meeting can be found here.
The Mobile Technologies in Clinical Trials Summit, 12 September, Boston
The Mobile Technologies in Clinical Trials Summit, taking place September 12th, Boston is designed for digital R&D professionals to get the best access to implementation strategies for applying mobile/digital tools to connect and empower patients, and obtain better outcomes in drug development/clinical research. Each year, we bring forward the latest experience-based case studies while presenting fresh ideas to advance fit for purpose, adoption and scale of remote digital solutions.
Kim Hawkins (Sanofi) joined the "REPORTING ON MOBILE/DIGITAL IMPLEMENTATION INITIATIVES AND DEMONSTRATING IMPACT" track to reflect on Trials@Home with a focus on the mobile/digital tools that will support RADIAL, and how they will impact/empower the patient.
Decentralised Clinical Trials – Hybrid Event | June 6 – 8, 2022
On day two of the Informa event, Fatemeh Jami (AZ) presented a CASE STUDY: Review of DCTs Through the Lens of QA
On-demand access to all presentation recordings from June 14th and you can find her slides here.
Conference – Decentralized Clinical Trials, June 6 – 8, Hyatt Regency Coral Gables, Miami, Florida
FEATURING Trials@Home's Kimberly Hawkins, Sanofi, on "Trials@Home Proof of Concept Study and a Sanofi Case Study".
Monday, 6 June 2022 2:00pm – 2:30pm EST/EDT (Eastern Daylight, GMT-4)
Explore the various ways you can decentralize your clinical trials and get to grips with the associated challenges and benefits with guidance from leading industry experts at Decentralized Clinical Trials, June 6-8, 2022 in Miami.
The event is free to attend In-Person for Pharmaceutical, Biotechnology and Medical Device industry professionals and spaces are limited.
Make the most of the opportunity to address all of your DCT questions in one place by securing a free pass today!
SCOPE Europe
20-21 April, Barcelona
IMI Trials@Home project aims to reshape clinical trial design, conduct and operations, by developing and piloting standards, recommendations, and tools for the definition and operationalisation of decentralised clinical trials in Europe. In its pilot study, RADIAL, we work with patients to assess and evaluate preferences and satisfaction around the concept of DCTs.
On 20 April at 10.15, Tanja Keiper (MERCK), Bart Lagerwaard (UMCU) and Maartje Roskams (IDF Europe) introduced the RADIAL trial and showed how we will approach questions on patient satisfaction on conventional compared to hybrid and DCT trial models, including specific patient-facing technologies.
DIA Europe
29-31 March, Brussels
29-31 March DIA Europe 2022 will take place in Brussels. The Trials@Home consortium is well-represented and there will be a dedicated "Decentralized Clinical Trials" poster session, during which research conducted by, amongst others, the EAGLE group will be presented; 'the mock ethics review' will be presented by Tessa van Rijssel and 'the interview study with regulators' will be presented by Amos de Jong. If you are planning on going to DIA Europe 2022, you are cordially invited to attend this session! Also, it may be a nice opportunity to meet some of the EAGLE colleagues in-person.
International Rare Disease Showcase
February 3, 2022 – online
At Beacon's International Rare Disease Showcase, Trials@Home joined the Main Stage session on 'The road to treatment: collaboration and innovation driving new rare therapies'. Here, Trials@Home presented about decentralised clinical trials to the conference's 430 attendees, piloting the RADIAL study, and how this can benefit the field of rare diseases. You can rewatch the presentation here:
DTRA Inaugural Annual Meeting
November 10-13, 2021
BOSTON, MA
Trials@Home will participate in a panel session during the DTRA Annual Meeting on the DCT-related work of other initiatives. This panel is scheduled for Friday November 12th 9am ET at the Boston Encore.
Confirmed Speakers from the following critical initiatives:
- TransCelerate: Hassan Kadhim (BMS)
- IMI Trials@Home: Kimberly Hawkins (Sanofi)
- CTTI: Reem Yunis (Medable)
- ACRO: Kushal Gohil (Parexel)
- SCRS: Casey Orvin (CenExel)
This session will be a conversation facilitated by Amir Kalali and Craig Lipset. Goal of the session will be: (1) ensure the diverse DCT leaders across DTRA are aware of the important work of other related initiatives and (2) begin to explore opportunities for "meta-collaboration" across initiatives where appropriate. The latter point builds on a regular cadence of leadership meetings DTRA has been holding with each initiative over the course of 2021 to ensure transparency and the potential for hand-offs.
DTRA is looking forward to a safe and rewarding opportunity to learn, network, and advance work together. Those joining in-person will be vaccine-verified with on-site antigen testing; all content will be streamed online to ensure learning opportunities are broadly accessible.
For more info click here https://www.dtra.org/registration