Request for Proposal
Submit your technology to Trials@Home

Trials@Home (EU/EFPIA Innovative Medicines Initiative – Joint Undertaking (H2020-JTI-IMI2) Trials@Home grant n° 831458) aims to reshape clinical trial design, conduct and operations, by developing and piloting standards, recommendations and tools for the definition and operationalisation of remote decentralised clinical trials (RDCTs) in Europe.
Request for Proposals (RfP)
In a collaboration with 31 international partners, the EU/IMI project called Trials@Home will check the feasibility of fully remote decentralised Clinical Trials (RDCTs). For the execution of these RDCTs, technologies are needed. Some of them are already familiar and/or contracted by the project partners, but other RDCT technologies are not and need to be introduced in this project and pilot study. We are looking for these technologies, and that’s why this Request for Proposals has been launched.
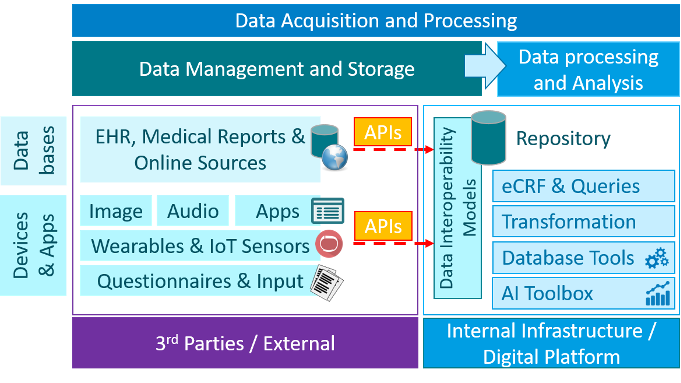
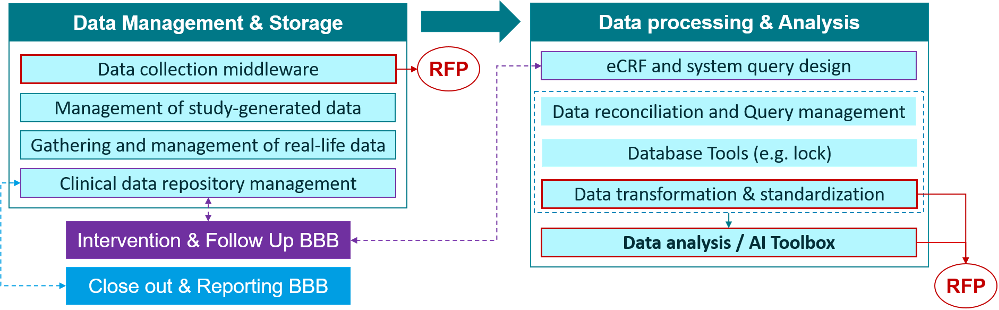
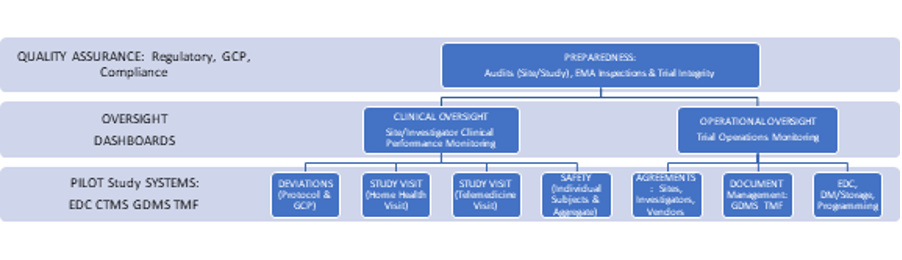
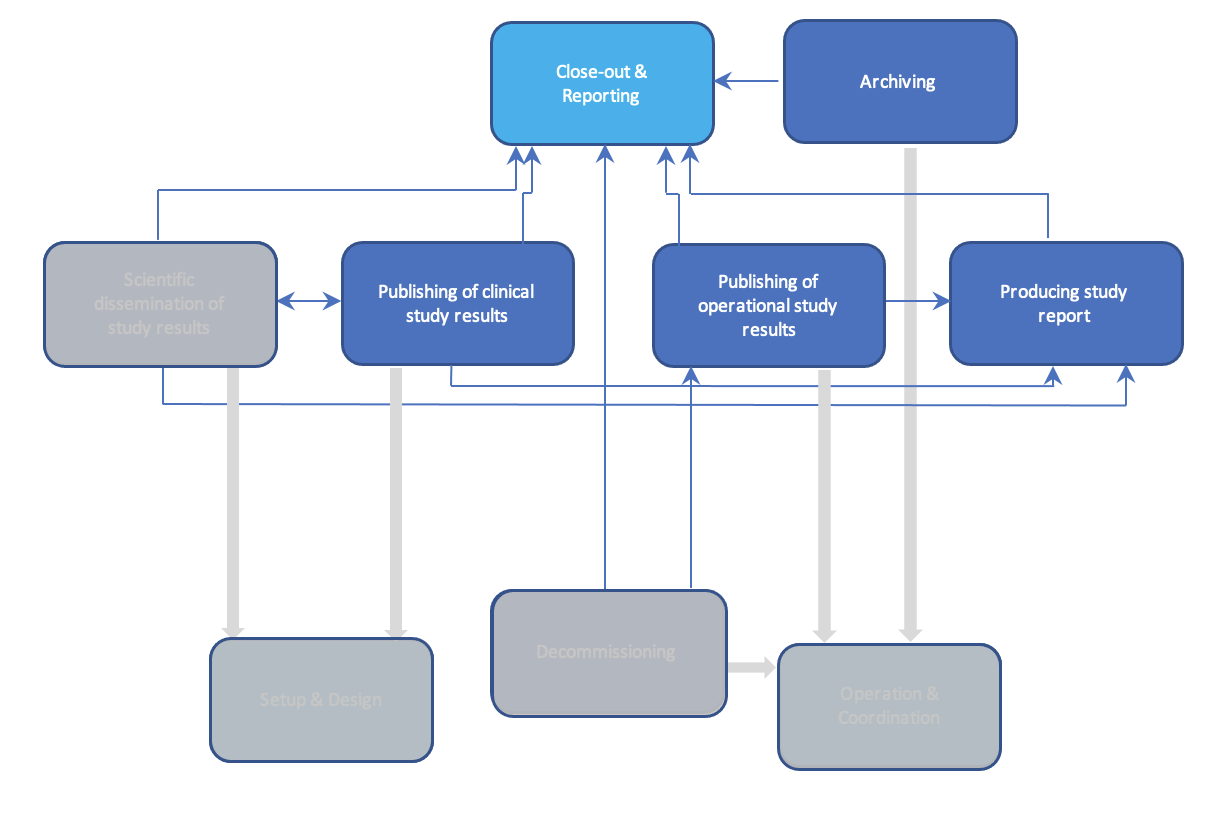
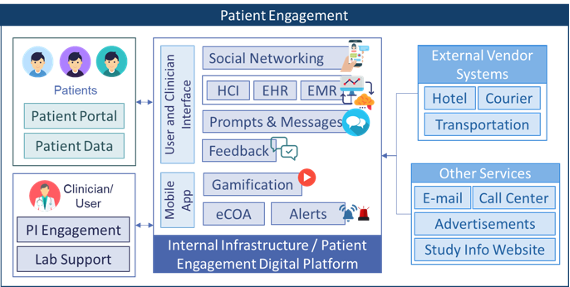
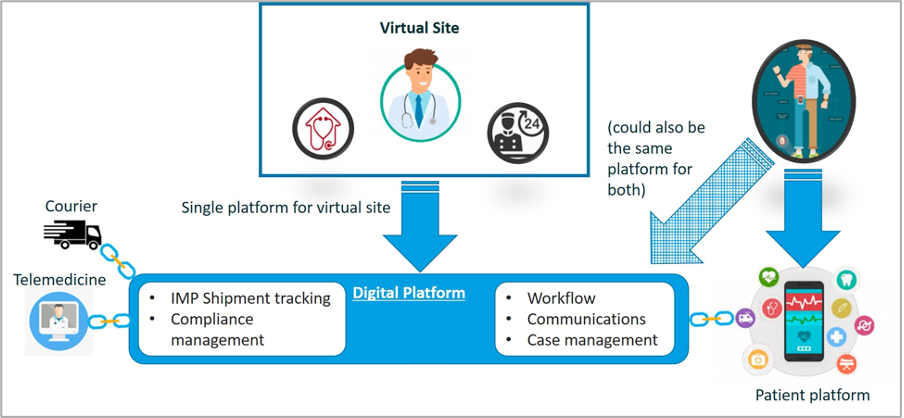
All the technologies are categorized in Basic Building Blocks (BBB) categories based on key functionalities within those building blocks, which are called activities. The scope of this RfP contains the following BBB’s / categories: Data Acquisition & Processing, Patient Engagement, Setup & Design, Closeout & Reporting, Operation & Coordination, Intervention & Follow-up and Recruit & Enroll. A description of these BBB’s and required technologies can be found in the ‘BBB profile’ tabs below or can be downloaded at the bottom of this page.
Submitting your proposal
We request you to create a proposal for the technologies that you are able to deliver. Please be very clear about the functionalities of your proposal: what part of the scope can be covered?
Also we would like to receive a total price, that shows at least the following components:
- Give a clear definition of the scope and detailed assumptions
- Present an overview with costs per line item (no lump sum)
- Per task indicate the estimated hours and role per task (including hourly rates)
- If applicable, detailed information about license costs per month/year/user etc
- Transparency when you outsource parts to third parties
- Overview of other costs and pass though costs.
You will be able to upload your proposal on the T@H website by clicking the ‘Submit a proposal’ button below.
After you have submitted a proposal for one or more of the requested technologies from the RfP, your proposal will be quickly checked on the knock-out criteria per relevant BBB. After a positive conclusion, you will receive a survey including instructions from Trials@Home. We kindly request you to fill in this survey, including remarks and documentation / proof to strengthen your answers, and return this form ultimately within 2 weeks.
Important Dates
- Webinar with further explanation of the project & RfP: 6 Jan 2021, 3 PM CET
- Deadline for submitting a proposal: 15 Jan 2021
- Deadline for returning self-assessment form: 29 Jan 2021
- Pitch + interviews: 16 Feb – 1 March 2021
Assessment process & criteria
After submitting a proposal and returning a completed survey, per BBB these documents per vendor will be assessed. First a check will be done on the knock-out criteria that apply. Then, if passed, the formal assessment will start. Per BBB, an assessment committee is composed, that will score all proposals including a self-assement survey, based on pre-defined quality criteria and vendor characteristics. You will find these quality criteria per BBB in the document ‘Quality criteria per BBB’.
The self-assessment will result in a ranking per BBB. Numbers 1, 2 and 3 of this ranking per BBB will be invited for a pitch & interview. Their offer will be discussed, and the assessment team is allowed to adjust their scores per vendor after this pitch and interview.
The vendor that ranks number 1 after the pitch and interview, and is assessed as fit for purpose, will be nominated as candidate technology for the pan-European pilot study.
Please note that, due to different unforeseeable circumstances, T@H always has the possibility not to award a contract.
FYI, upon award of services, the attached contract template will be completed and executed. The standard terms and conditions are non-negotiable.
Questions
All interested vendors may ask questions during this RfP. You can do so by sending your question per e-mail to: trialsathome@umcutrecht.nl.
Questions will be collected and answers will be – open to everybody – uploaded on the Trials@Home website.
Webinar Video
Webinar Slides
Click on the image to download the slides
FAQ
Click on the image to download the FAQ document. This document will be updated regularly with all questions received prior to the submission deadline. Current version dates 13 Jan 2021
CDA Template
Please click here to download a signed copy of our CDA to add to your submission if you prefer doing so