Project Updates

Digital Literacy in DCTs. What is asked of trial participants?
Webinars Trials@Home – Digital Literacy in DCTs. What is asked of trial participants? 2 March 2023, 15.30-16.45 CET DCTs offer a more patient-centric approach in clinical [...]
ACT EU Recomendations
ACT EU Recommendations for DCTs Utrecht, March 1, 2023 What is ACT EU? The Accelerating Clinical Trials in the EU (ACT EU) initiative, launched in January 2022, aims to develop the EU further as a [...]
Trials@Home Annual Meeting #3 Wuppertal 26 & 27 September
Trials@Home Annual Meeting – 26 & 27 September 2022 Wuppertal & Online The 2022 Trials@Home Annual Meeting took place in Bayer’s facilities in Wuppertal, where we had two full [...]
Trials@Home – Where are we two years down the road?
Webinars Trials@Home – Where are we two years down the road? 1 December 2021, 15.00-16.00 CET Two years ago, over 200 people from more than 30 [...]
Full steam ahead for RADIAL trial, all CTIS approvals are in, and the UK is on board too
Full steam ahead for RADIAL trial, all CTIS approvals are in, and the UK is on board too. Utrecht, 29 November 2023 After the good news that the Danish authorities approved [...]
RADIAL is approved in Denmark!
RADIAL is approved in Denmark! Utrecht, November 2, 2022 On the 5th of September the team submitted all the necessary documents related to RADIAL for the second part of the CTIS [...]
PRESS RELEASE: BIOCORP: AARDEX® Group and BIOCORP recruited by Trials@Home – Decentralized Clinical Trial Centre of Excellence
PRESS RELEASE BIOCORP: AARDEX® Group and BIOCORP recruited by Trials@Home - Decentralized Clinical Trial Centre of Excellence July 21, 2022 The AARDEX and BIOCORP teams are outstanding collaborators, [...]
RADIAL CTIS Part I approved & MHRA Submission done!
RADIAL CTIS Part I approved & MHRA Submission done! Utrecht, 21 July 2022 Very good news: Trials@Home has received approval from the regulators on the CTIS part I submission of the RADIAL [...]
Public Report Trials@Home Semi-Annual Meeting 7-8 June 2022, Brussels
Trials@Home Semi-Annual Meeting Hybrid, 7 & 8 June 2022 On June 7 and 8, nearly two and a half years since the kickoff meeting in Paris, Trials@Home hosted a [...]
Decentralised Clinical Trials are all about participant centricity
Decentralised Clinical Trials are all about participant centricity Utrecht, May 20, 2022 On 20 May 1747, James Lind started what is often considered the first randomised clinical trial aboard [...]
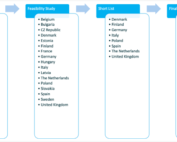
RADIAL Country selection process
RADIAL Country selection process To select the countries where the pan-EU RADIAL pilot study will be conducted, a multi-step approach was used while considering a good spread across Europe [...]
RADIAL submitted in CTIS
RADIAL submitted in CTIS Utrecht, March 28, 2022 We are pleased to inform you that we have submitted the RADIAL study under the new Clinical Trial Regulation using [...]
Public Report Trials@Home Annual Meeting – 2 & 3 November 2021
Trials@Home Annual Meeting Virtual, 2/3 November 2021 On November 2 and 3, Trials@Home organised, in virtual format, its major annual event. More than 150 public and private partners and [...]
First vendor for RADIAL proof-of-concept-study contracted
First vendor for RADIAL proof-of-concept-study contracted Utrecht, December 22, 2021 As discussed in our latest webinar, we have selected the full tech package for the RADIAL study and are [...]
Trials@Home Newsletter – November 2021
Trials@Home Newsletter Utrecht, November 22, 2021 Trials@Home - A vision for transforming clinical trials RADIAL, a test run for clinical trials of the future The [...]
Trials@Home – A Public Call for Proposals to build an end-to-end DCT Tech Package – The RfP explained.
Webinars Trials@Home – A Public Call for Proposals to build an end-to-end RDCT Tech Package – The RfP explained. Wednesday 6 January 2021, 15-16h CET For [...]
Finalised list of KPI’s to be used to qualify and quantify the flow of activities in the pilot (D3.2)
Finalised list of KPI’s to be used to qualify and quantify the flow of activities in the pilot (D3.2) Lead Contributors: UMCU, Sanofi July 20, 2021 Abstract [...]
General Practitioners Survey
Are you a general practitioner? Do you have 5 minutes for us? Please complete this survey and help us better understand how we can help you better understand what RDCTs are all about. [...]
Open call for additional technology partners (D6.5)
Open call for additional technology partners (D6.5) Lead Contributors: Nathalie Vigot (UMCU PMO) June 21, 2021 The Trials@Home pan-European pilot (pan-EU pilot) study aims at comparing traditional clinical trial [...]
Public Report Semi-Annual Meeting April 2021
Trials@Home celebrated its annual meeting’s extra spring edition Virtual, 23 June 2021 After nearly 2 years of intense work and study in the field of decentralised clinical trials, more than [...]
Mapping and analysis of the EU legislation on Remote Decentralised Clinical Trials (D4.1)
Mapping and analysis of the EU legislation on Remote Decentralised Clinical Trials including legal, regulatory, ethical and stakeholder recommendations for the conduct of the pan-EU pilot (D4.1) Lead Contributors: Yared Santa-Ana-Tellez, Utrecht University - Helga [...]
Patient Survey
This survey is now closed. If you like to know the results or participate in future surveys, please contact Petra Naster
Launch Webinar: Innovative Medicines Initiative Trials@Home A Centre of Excellence in Remote and Decentralised Clinical Trials
Webinars Innovative Medicines Initiative Trials@Home A Centre of Excellence in Remote and Decentralised Clinical Trials Wednesday 24 June 2020, 11-12 CET Trials@Home aims to reshape clinical [...]
Request for Proposal
Request for Proposal Submit your technology to Trials@Home Trials@Home (EU/EFPIA Innovative Medicines Initiative - Joint Undertaking (H2020-JTI-IMI2) Trials@Home grant n° 831458) aims to reshape clinical trial design, conduct and [...]
Trials@Home Annual General Meeting 5-6 October 2020 – Public Report
Trials@Home Annual General Meeting Public Report 5-6 October 2020 September 2019, the great and grand kick-off meeting for the Trials@Home project in Paris. A big project, a large group [...]